���ı��⣺Formation of CaCO3 hollow microspheres in carbonated distiller waste from Solvay soda ash plants������Solvay�����������Һ����CO2�ϳ�CaCO3���������о���
�ڿ���Frontiers of Chemical Science and Engineering
���ߣ�Wenjiao Xu, Huaigang Cheng, Enze Li, Zihe Pan, Fangqin Cheng
����ʱ�䣺01 Aug 2022
DOI��10.1007/s11705-022-2173-z
�����ӣ�����˴��Ķ�������
�о��侰������
2021��ȫ�����Լ5900����������Solvay���Ƽ����ռ��45%���Solvay���ÿ����һ�ִ���ᷢ��10 m3��ǿ����������Һ�����2021��ȫ��ͷ�����2.655��m3��������Һ�����˴�����������Һ�����̬������ɼ����Σ�����Ϊ�����价����������������������Ϊ������Һ�ȹ�ҵ��Һ������Ͷ���˴��������������Ը�����������о������������Һ����CO2�ļ���·������ֳɺϳɳ���ֵ������—CaCO3�������������ǿ��������Һ�����ľ������������“̼��塢̼�к�”���侰�����Ϊʵ��������Һ��Ч��̼�ṩ�˽�������
�о����ݼ���Ҫ����
���ɼ�ʵ��Ҫ�������ͼ1��ʾ�����ģ��������Һ��ͨ��CO2�ϳɳ�CaCO3���������������������ͼ2��ʾ�����Ϊ̽�����Ľṹ���γɻ����������̽����������Һ�е�Ca(OH)2�����Բ����γɵ�Ӱ������Ƿ���Ϊ���Ľṹ��ģ������Ȼ��ͨ�������ܺͽ��������ļ����������˿�������ܵ��γɻ���������������������Һ����CO2����̼��ƿ�����Ĺ��̽����˹�ҵ������ƺ���Ӫ����ְ��������õ������½��ۣ���1��ͨ��ʵ��̽������û��Ca(OH)2�����ķ�ӳ��ϵ�����γɷ���ʯ�Ϳ��Ľṹ����ɴ˿ɼ�������Һ�е�Ca(OH)2�����Կ�������γ�����Ҫ�����������Ϊ��ʼ״̬����ϵ��Ψһ�Ĺ������Ca(OH)2�����ܿ����ṩ�˽ᾧ���������ͼ3�������ô���Ca(OH)2���������ǿ��Ľṹ��ģ��������ͨ��Pitzerģ�Ͷ���ϵ�л�����ӵļ���ó��Ľ����Ƿ��ϵ��������2��ͨ�����Ӷ���ѧģ������˾����������̵Ľ��������ͽ����������ͼ4������߲�ֵ�ı仯���ö�Ӧ������ò���ı仯����������������CaCO3��������γɻ���—���������Ostwald�컯���������ͼ5�������3��ͨ��������Һ����CO2����̼��ƿ�����Ĺ�ҵ������ƺ���Ӫ����ְ����֪�ù������������ǿ��е������������ͼ��ͼ6��ʾ������յľ�Ӫ����Ϊÿ��39.9Ԫ�����

ͼ1 ʵ��װ�ü�����ͼ
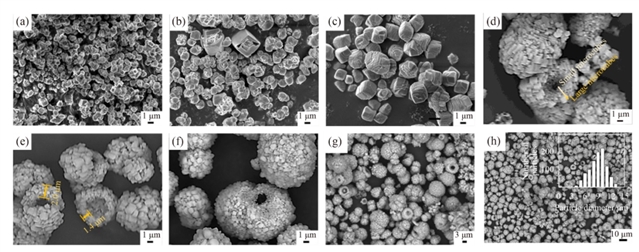
ͼ2 ̼��ƿ�������������̵�SEMͼ

ͼ3 ������Һ����/��Ca(OH)2����̼��Ʋ����SEM��XRD��FTIRͼ

ͼ4 ���������ͽ����ܵķ��Ӷ���ѧģ��ʾ��ͼ
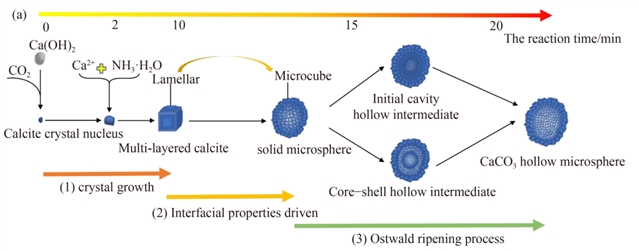
ͼ5 CaCO3���������������
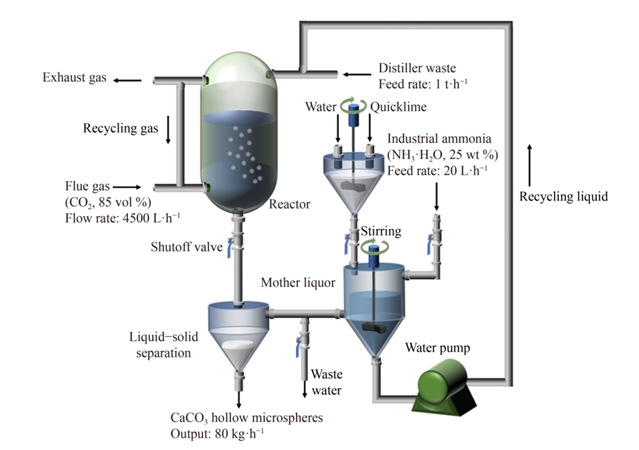
ͼ6 ������Һ����CO2�Ĺ���·��ͼ
�����
�����ַ�����—������Һ��CO2Ϊԭ���Ʊ��˸�ֵ����CaCO3�����������̽�������������γɻ���������˽����ʯ��CaCO3����״�仯�ܽ�������/�����ܵ�������������CaCO3�������������һ�����������Ostwald�컯�������������������������ƺ���Ӫ����ְ�������������ù������������ǿ��е��������ؽ����“Formation of CaCO3 hollow microspheres in carbonated distiller waste from Solvay soda ash plants”Ϊ������ѷ�����Frontiers of Chemical Science and Engineering�ϣ�DOI: https://doi.org/10.1007/s11705-022-2173-z�������
�����Ŷӽ���
���Ľ�����һ���ߣ����ɽ����ѧ2018����ʿ�о�������о�ƫ��Ϊ������Һ����Դ�������
�ɻ��գ�ͨѶ���ߣ������������������������ˮ����ϵ�ѧ��Ӧ�÷��������о��������Ҫ��չ�����ᾧ/��ѡ�������ο���ȡ�Ƽ�þﮡ���/Ĥ����ˮ������ǿ����Լ����θ߸Ʒ�ˮ�������õ��о���������֮�йص�̫���ܼ����ȼ������á�������̼��������Դ�����á�������̼������ʯ��/������Һ�Ʊ��������ϡ�þ﮹�Ч�����ϲ��↑��������������ж���ĺ����ι��̼���ˮ�������ֳ�����������ó��ڹ�����ͼ������ƵĽ�ѧ��ʵ������ѷ�������60��ƪ�������SCI/EI��¼30��ƪ��������Ȩר��10�������
�̷��٣�ͨѶ���ߣ������������������ɽ����ѧ��У��������һ���������ú̿��������Դ����Ч���ü����ص�ʵ���ҡ�CO2��������Դ�����ý����������о�����������������“�����Ͱ�ǧ���˲Ź���”��ѡ�����ɽ��ʡ“����ѧ��”��Ƹ����������ܹ���Ժ�������ר���������ô���ú����������Դ����ֵ���ú���Ⱦ���ƵĹ��̻�Ӧ���о����Χ��ú̿���ɡ�ú��ұ�硢ú�������̷����ķ����������ú��ʯú����Դ�����ȼ�������ú�ҡ�ú��ʯ���м�Ԫ���ݼ��������ú���̷����ϻ����õȷ��������ϵͳ�о���ȡ���˴����Խ�����Ϊ�ҹ���ҵ�̷ϴ������ɡ���ֵ���ú͵���ֵú������ҵ�����Ľ���������һ��Т��������Ⱥ����ֹ����ص��з��ƻ�������“863”�ƻ���Ŀ�����ҿƼ�֧�żƻ���Ŀ�ȹ��Ҽ���Ŀ9�����ʡ�����ش���Ŀ15�����������ѧ������200��ƪ������Ȩר��50������������ר��5�����������ط��߶�3������6��Ƽ����ʵ�ֹ�ҵ��������Ե�һ����˻�ù��ҿƼ��������Ƚ�2�����ɽ��ʡ�Ƽ�����һ�Ƚ�4��������Ƚ�3��������Ƚ�3������������� “ȫ����һ�������”��“ȫ�����˺�����”��“ɽ��ʡ��һ�Ͷ�����”��������ν�����
ժҪ
For decades, distiller waste and CO2 were not the first choice for production of high valued products. Here, CaCO3 hollow microspheres, a high-value product was synthesized from such a reaction system. The synthetic methods, the formation mechanism and operational cost were discussed. When 2.5 L·min–1·L–1 CO2 was flowed into distiller waste (pH = 11.4), spheres with 4–13 μm diameters and about 2 μm shell thickness were obtained. It is found that there is a transformation of CaCO3 particles from solid-cubic nuclei to hollow spheres. Firstly, the Ca(OH)2 in the distiller waste stimulated the nucleation of calcite with a non-template effect and further maintained the calcite form and prevented the formation of vaterite. Therefore, in absence of auxiliaries, the formation of hollow structures mainly depended on the growth and aging of CaCO3. Studies on the crystal morphology and its changes during the growth process point to the inside–out Ostwald effect in the formation of hollow spheres. Change in chemical properties of the bulk solution caused changes in interfacial tension and interfacial energy, which promoted the morphological transformation of CaCO3 particles from cubic calcite to spherical clusters. Finally, the flow process for absorption of CO2 by distiller waste was designed and found profitable.